BRUGADA SYNDROME
Brugada syndrome or Brugada disease is an inherited heart disease that belongs to the group of inherited channelopathies. The first cases were reported in the early 1990s by the Brugada brothers who gave it their name. It is caused by an abnormal function of the ion channels that regulate the electrical impulse of the heart.
The heart rate is generated by a set of specialized cells which cause regular contractions. The contractions are activated by ion currents flowing through the cardiac cell membranes. The ion currents travel through specialized channels called ion channels. There are many ion channels, all of them controlled by different genes. The main channels are sodium (for sodium ions), potassium (potassium ions) and calcium (calcium ions) channels. A gene mutation affecting one of the channels is likely to cause abnormal electrical impulses of the heart (it is called membrane action potential anomaly). This is how diseases described as hereditary channelopathies such as Brugada disease and others (congenital long QT syndrome, …) function. Brugada disease is generally (but not exclusively) linked to a genetic abnormality in the sodium channel (sodium). There are several genes and mutations responsible for Brugada disease. Not all of them are known yet.
In the vast majority of cases, Brugada disease is a completely asymptomatic, lifelong condition. However scarce, some patients with a severe form may experience heart rhythm disorders. They are sometimes severe ventricular rhythm disorders (ventricular tachycardia, ventricular fibrillation) which manifest themselves by palpitations, transient loss of consciousness (syncope) or even by cardiac arrest (sudden death). However, these “rhythmic accidents” often occur in specific conditions such as taking a contraindicated medication, a fever peak or alcohol intake.
There are 2 possible ways to diagnose Brugada disease.
The first, and by far the most common one, is the electrocardiogram (ECG). The ECG makes a definite diagnosis only when it is typical. Indeed, a patient with Brugada disease may show varying degrees of electrocardiographic modifications, ranging from the most marked form called type 1 Brugada on the one hand, to the total absence of anomaly (normal electrocardiogram) on the other hand. But there are intermediate forms called type 2 and type 3 Brugada aspects.
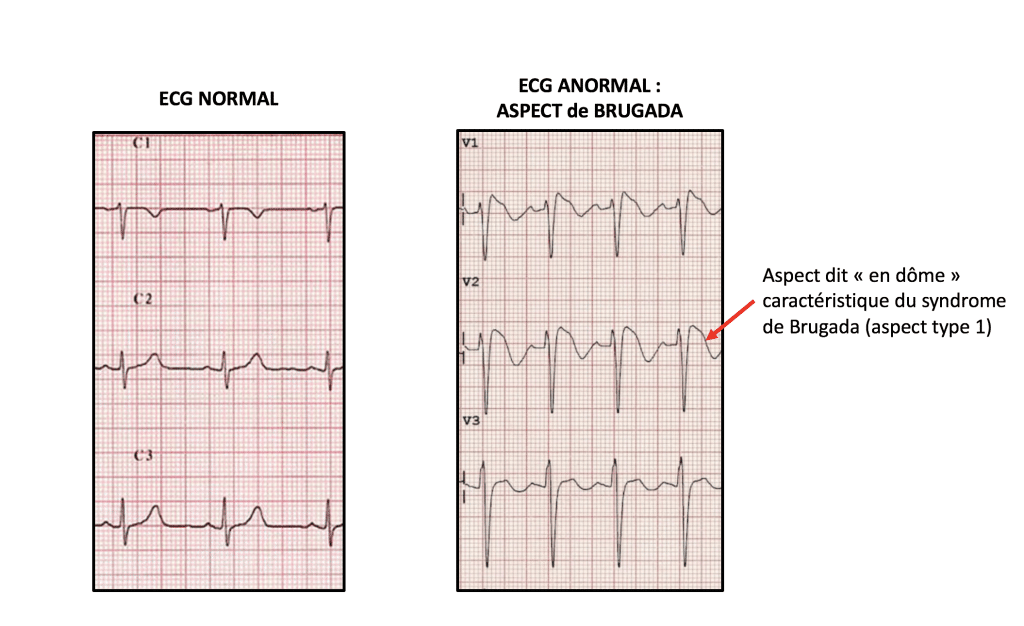
Since only type 1 allows a definite diagnosis of Brugada disease on the ECG, intermediate forms (type 2 and type 3) can translate either an attenuated form of the disease, or a variant of normality. Therefore, we use a pharmacological diagnostic test with anti-arrhythmic drugs (most often an ajmaline test) to pinpoint the appropriate diagnosis. The Ajmaline test is a pharmacological one, usually performed on an outpatient (who spends a half-day at the hospital). The patient is administered intravenously a drug (usually an Ajmaline-type anti-arrhythmic, or sometimes flecainide) while performing ECG recordings. It then makes it possible to confirm or deny the diagnosis of Brugada disease. It is so practiced in patients who have a suspicious but not diagnostic aspect on the ECG (type 2 or type 3 Brugada aspects).
The second possible way is the genetic test. It is only carried out only under 2 conditions:
Once Brugada disease has been diagnosed, the management of the patient focusses on several lines of action to prevent any heart rhythm disorder:
As mentioned above, heart rhythm disorders in Brugada disease are rare but can be life-threatening. They often occur as a result of triggering factors that need to be detected:
As Brugada disease is a genetic disease, tests should be carried out to find the mutation responsible (there are several, but not all of them are known yet.). A blood sample is necessary for the test but it can take months, if not years, to get the results. Once the gene involved and its mutation have been documented, a genetic screening will be offered to the close relatives. The aim is an early diagnosis for an optimized follow-up as soon as possible.
There is no cure for Brugada disease. Its management consists mainly in assessing the risk profile of each patient. To do this, different elements can help the rhythmologist.:
In patients deemed most at risk, an implantable cardioverter defibrillator will be proposed. The aim is to protect the patient from sudden death due to a severe ventricular rhythm disorder. Other treatments are also being studied but have not yet proved their effectiveness (drug treatment with quinine, radiofrequency ablation).